OUR SERVICE
A solution is only as good as the weakest part. To find out which are the possible risks and what to do to make a solution more safe is necessary to develop the optimal solution for your application. And if there is a defect, then you need to get support right away, without any delay. Werth Systems is able to support you in all of these issues.
Werth Systems is a full service company for solutions in patient treatment areas. We have a modular design for our devices.
This helps you to reduce the time and the costs for the development and shorten the time to market stage.
Certification, risk management, EMC, electrical safety, evaluation of thermal and mechanical properties, long time availability and creation of parts lists are our daily business. We share with you our uncomplicated and fast customization service.
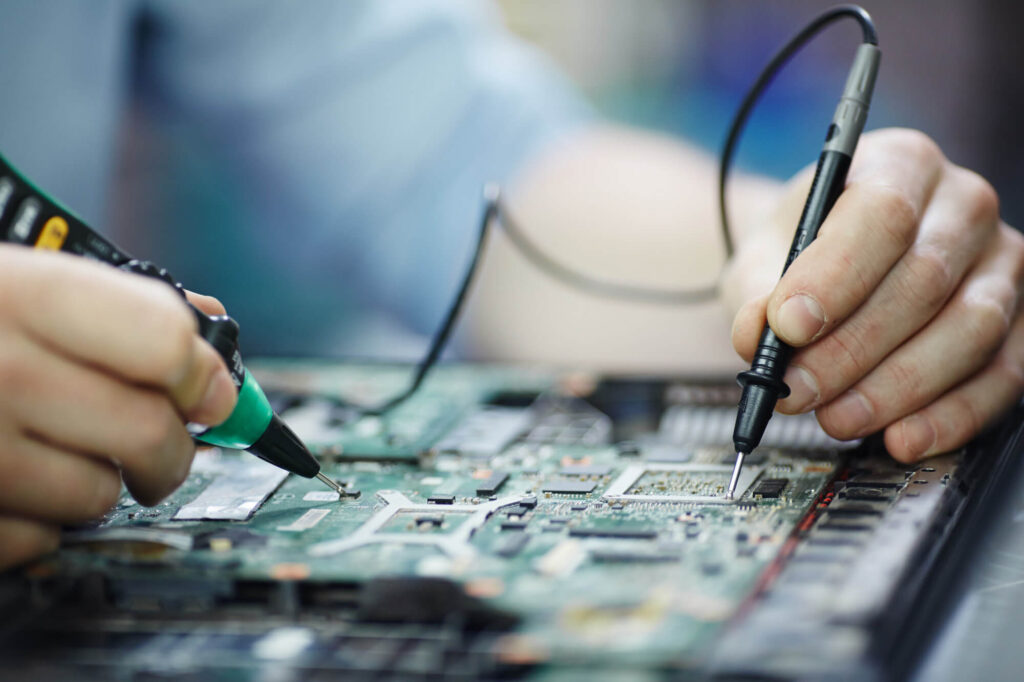
Personal consultation
We are certified Medical Device Consultants with longstanding experience in many European countries, in conjunction with our local partners. We have a solid and successful operations track record in a variety of customer needs and solutions. Furthermore, we are reliable experts and successfully managed extensive R&D projects with consistent quality and delivery within the required timelines.
OEM / ODM Development
A standard product is not always the perfect solution for fulfilling the given requirements. Our aim is to assist you in all project stages or create a complete new solution for you from the scratch. In case of larger projects, we also provide on site support in order to offer our customers the best available assistance.

Warranty extension
Our products may be purchased with two to five years of warranty and on various service levels. The type of warranty is depending on the product's and consented agreement. The warranty can be either a normal factory warranty or an extended service level including replacement devices.
after sales service
Depending on the chosen warranty within the purchase, we offer different options of after sales services. Important for us, not depending on the choosen warranty, is a fast response and solution for the avoidance of disruption of treatment areas or unnecessary downtime.
Our full service house
Werth Systems is a Full Service House for solutions in patient treatment areas. We have understood that the resources in hospitals and at Medical Device Manufacturers are decreasing constantly, and everything can’t be done in-house. In addition, we know that the policies tend to differ greatly between different applications and system makers. We help you to develop and to produce exactly the right solution for your need. Certification, Risk Management, EMC, electrical safety, evaluation of thermal and mechanical properties, availability, and creation of bill of materials are our daily business. You don’t have to start from the scratch anymore, and you can save a lot of time and money if you decide to use our expert knowledge for your projects.
However, it is not effective that hospitals or medical device manufacturers provide ICT solution development and IT maintenance for the patient treatment Areas, because that is not their main business. In addition, the equipment acquisitions can be challenging when done internationally. Either the product doesn’t exist, or the selection is so wide, that a suitable and high-quality product is very challenging to find.
We also offer consultancy and pre-sales services. Different types of carts, wall, and table mounts require installation space as well as understanding of their weight limitations. The law of health care equipment and supplies:
MDR – General requirements for professional use
The professional user has to ensure that:
- A person who uses a health care device, has the training and experience required for its safe operation;
- A label and instructions for safe operation are required on the device or accompanied by it;
- the device is used by the manufacturer in accordance with the purpose and given instructions;
- the device is maintained and serviced in accordance with the manufacturer’s instructions;
- the location is suitable for the safe use of the device;
- The immediate proximity or in connection with the device of the medical equipment, building components and structures, equipment, software, or other systems and objects do not compromise the performance of the device or of the patient, the health of the user or any other person; as well as
- the installation, maintenance, and repairs of the device can be done only by someone who has the necessary skills and expertise.
Critical treatment areas such as the operating room, critical care, anesthesia, etc. set the requirements for hygiene and for the method of installation. The nurse is not in charge of cable ties. Small spaces and on the ground, in the middle of patients, is not the place to start opening packages and reading the installation manuals.
Werth Systems has developed processes in which the setup time in the treatment areas are reduced significantly. Device configurations are planned and prepared well in our production facilities. We will transport an almost finished device pre-configured at the scheduled time frame on location. In this way, we disturb the patients and treatment work as little as possible.
"All our WERTH SYSTEMS Medical Computers are subjected to our own design and development process, which we based on the IEC60601-1 specification, to ensure a flawless system".
Dirk Werth, Founder & Managing Director
Discover our Medical Box PCs and Panel PCs

WERTH SYSTEMS CMP-2450
WERTH SYSTEMS CMP-2450 All-in-One-PC is our 23,8″ fanless and extendible medical panel pc in a durable and cleanable aluminum case with PCT-Touch-Glass front and a PCIe x16 Slot for additional Cards.

WERTH SYSTEMS XE-850 Medical PC
The WERTH SYSTEMS XE-850 Medical Computer is our compact, fast and fanless Medical Box PC in an aluminum housing especially designed for hygiene sensitive areas and it is a perfect solution for e.g. PDMS applications.

SMART-MED PXE-2250 All-In-One-PC
The WERTH SYSTEMS PXE-2250 is fanless 21.5″ medical Panel PC in a closed aluminum case and with full flat IP65 PCT-Touch-Glass-Front. Made for those looking for a high-quality, robust device without a lot of bells and whistles.

Werth Systems is a manufacturer and distributor of IT-Solutions for the Healthcare Market. Our technicians and engineers have more than 30 years of knowledge in fanless computerdesigns and they are specialized in developing of safe systems for the patient environment.
May we help you?
Our experts will be happy to help you with any questions, queries and problems you may have. Please contact us by phone or e-mail from Monday to Friday from 8:00 am to 5:00 pm CET.
- +49 (0) 5223 / 94038 - 40
- sales@werth-systems.com

